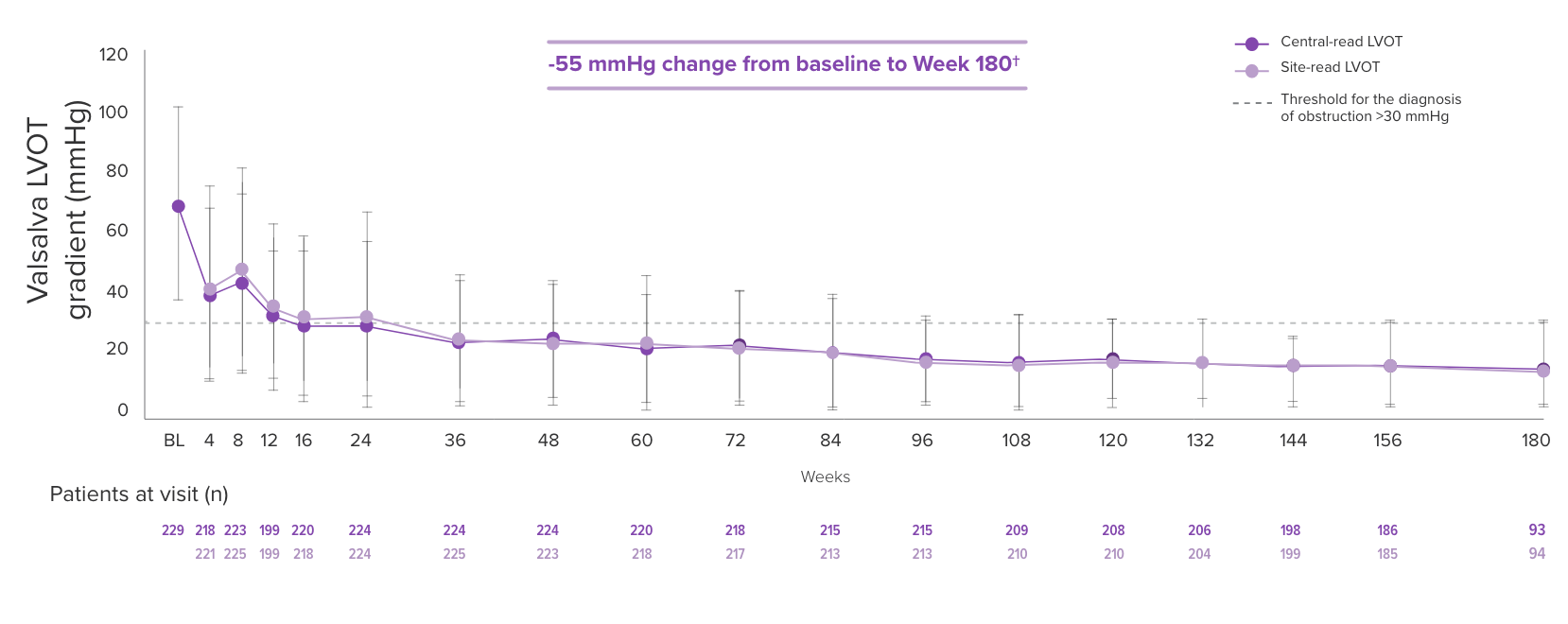
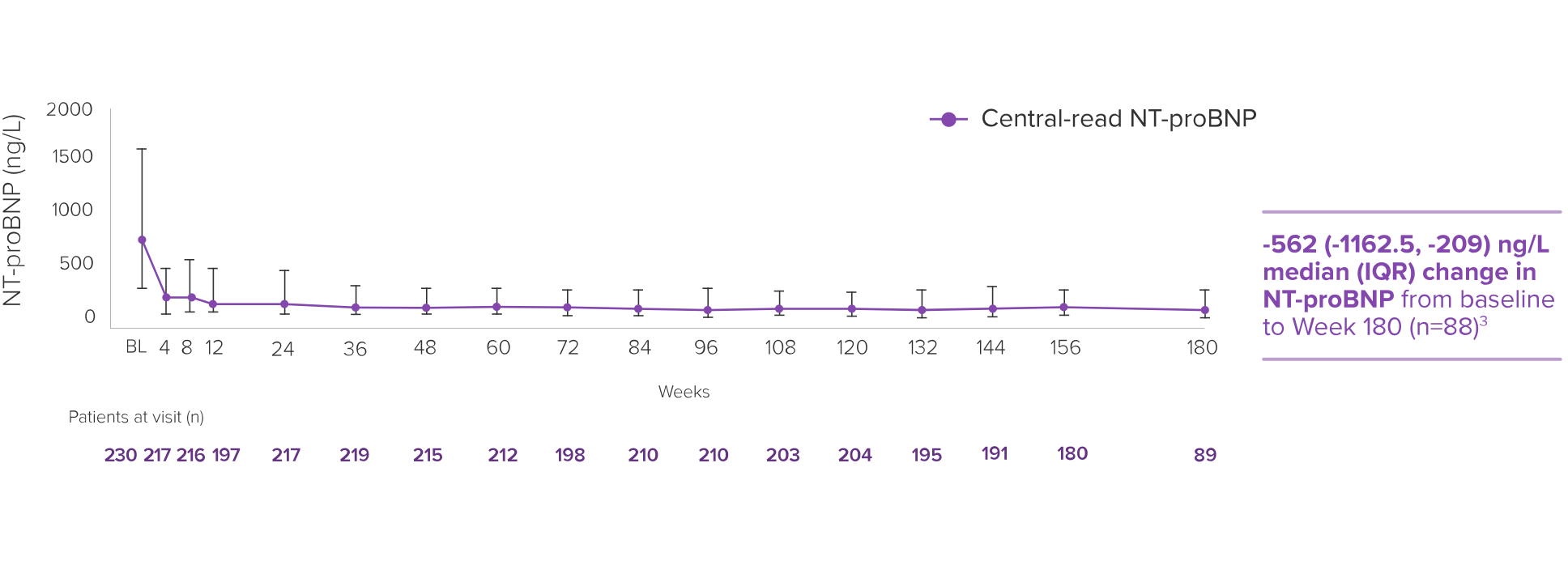
The EXPLORER-LTE cohort of the MAVA-LTE trial sought to evaluate the safety and efficacy of CAMZYOS starting at 5 mg in adults who had completed the EXPLORER-HCM trial.* The EXPLORER-LTE cohort (n=231) is a single-arm study without an active comparator.3,5,6
Dose adjustments could be made at Weeks 4, 8, 12, and during subsequent visits based on the patient’s Valsalva LVOT gradient and LVEF, except for Week 24, in which the patient’s stress TTE was used.† Unscheduled dose adjustments could occur at Week 24 and every 12 weeks thereafter. Treatment was temporarily interrupted if LVEF <50%. Mean time from EXPLORER-HCM end of study to Day 1 of EXPLORER-LTE was 66.5 days (range: 3-359 days).3,5,6
| * | Patients enrolled in EXPLORER-LTE were part of either the CAMZYOS or placebo arm in the EXPLORER-HCM trial.3,5,6 |
| † | This is not consistent with the current approved dosing schedule per the US Full Prescribing Information.3 |
TTE=transthoracic echocardiogram.